Change is only continuous event, at least this proverb is holding true literally in the laboratory operations. Many of the changes in recent time was due to the way the labs are governed by regulatory guidelines.
NABL had made it essential for labs to provide unique lab report in the prescribed series format for all the deliverables that are covered in the accredited. The edict has been adapted and included in lab operations since Sep’18. As NABL promised this was not the end, other changes followed. Two major changes have hit Laboratory operations:
Change in Decision Rule Outcome methodology
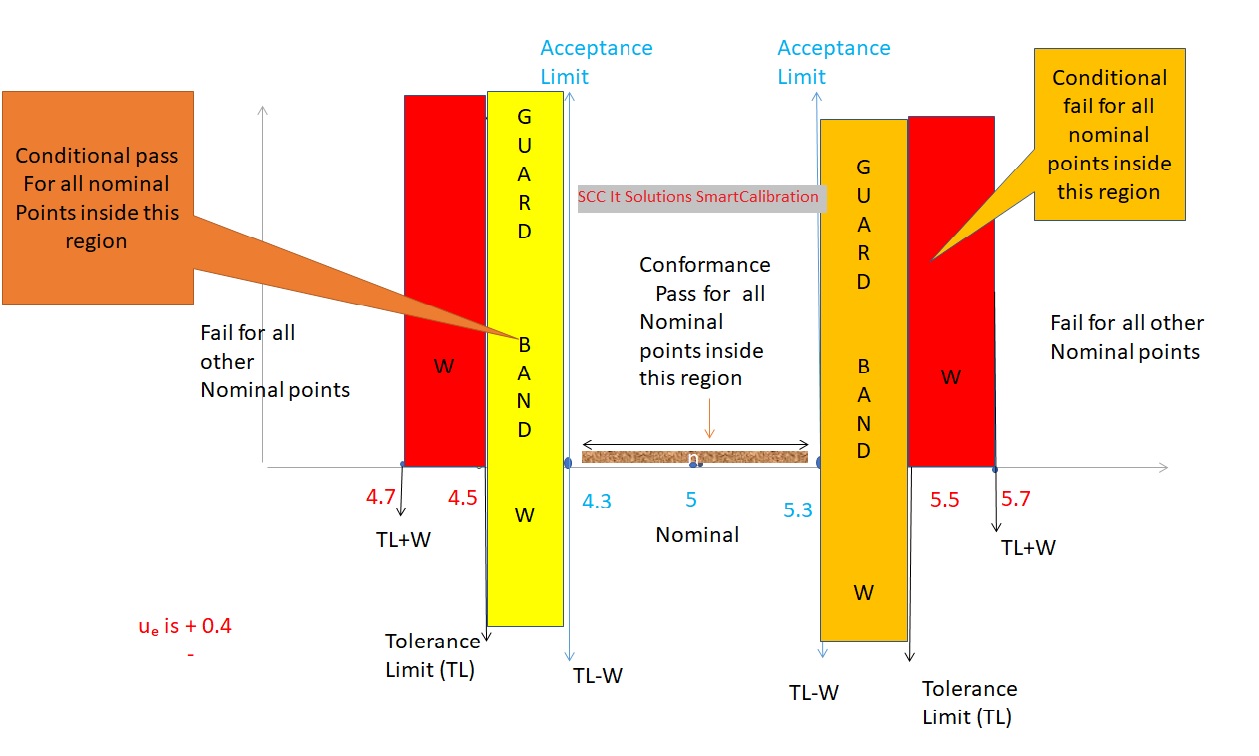
With the adaptation of ISO17025:2017 guidelines, it has become imperative for labs to provide decision rule outcome on the basis of newly laid out criteria. The newly laid out criteria requires uncertainty of the lab or object measurement method to be considered in the process of arriving at outcome. Two additional indicator (“Conditional Pass” and “Conditional Fail” ) are made part of the outcome result.
Impact of this requirement is though vast but limited in the sense that it is still optional and decision rule outcome is to be given only if customer seeking service ask for the decision rule outcome. For testing laboratories, impact is minimum as conformity was already being reported where ISO guidelines are established. However for Calibration Laboratories, conformity was given only when customer sought their views on it as ISO guidelines were given only UUC that falls within the domain of legal metrology and not applicable in general on other instruments of measurement or devices.
NABL Logo (Accrediation Body Logo) is essential just like ULR for accredited scope
Why this is a big change, earlier labs in India were affixing accreditation body Logo on only selected report/test certificates as they claimed the service receiver has not insisted or requested them to test as per laid out processes by NABL or ISO17025. Hence they were not publishing their deliverables without logo and that also meant the process through which the deliverable are formed were not conforming to ISO17025 guidelines.
This practice of omitting NABL Logo, probably caused degradation of quality of deliverable with the advantage of efficiencies to labs. NABL now has now taken away the flexibility of choosing whether to print NABL Logo from the labs and service receiver and have issued another circular that states clearly following points
i. NABL Logo to be affixed on all deliverables published by the accredited labs for all accredited scope and it must be carrying a ULR (unique Lab Report number ) as per the format prescribed by NABL in 2018 circular.
ii. Where there are some or all parameters or points, out of scope; in that case, a separate deliverable need to be issued that shall not be carrying NABL Logo
iii. Records of all jobs performed need to be maintained and are audit-able in due course of time.
Maintaining two registers one register for jobs where LOGO was fixed earlier were smaller and the other without Logo were bigger and larger. These two register shall still need to be retained however the scale is likely to tilt toward More jobs with NABL Logo and less without Logo.
So having discussed changes above, the question arise now is, What shall the Lab do to maintain their compliance and governance overhead while maintaining the margins and efficiency?
What the change would require from labs are as follows; It is obvious one of the driving criteria for the change shall be to stay efficient and control the cost of improvement in quality and governance overhead.
1. Lab may cut their scope deliberately for job types that are rare and come occasionally thus removing unnecessary fat. This has a limited appeal and benefits are limited as well.
2. Increase in cost is passed onto customer, this is highly unlikely due to intense competition and thirst for more business, customer is likely to change supplier rather than accept the increased cost.
3. Labs need to calculate each and every calibration point and parameter, for e.g. for Calibration Labs, they need to maintain spreadsheet for every calculation (from environment correction and Uncertainty) reported. So the manual/clerical effort required post technical Calibration would increase and so would be the job of engineer who would be reviewing the data and calculations for sanity and accuracy respectively.
In order to do calculations, calibration labs need to adapt to more automated excel sheet . But that is not likely to solve the problem entirely as putting data in spreadsheet for calculation is first step, In next step, lab have to pull the data from this spreadsheet in to MS Word or equivalent word processor to publish Certificate in PDF format. The availability of cheap labor in India may make this look easier from economic cost but it is obviously adding a cost on top of existing cost of operation. Since the data is passing through two manual steps, probability of leakage in quality is significantly higher and any detection of these kind of leakage could endanger accreditation of the Lab.
Therefore the better solution would be to adapt a Calibration Lab Software that support automatic calculation (such as SmartCalibration from Smallcapcrm IT Solutions Pvt. Ltd.).
SmartCalibration seek data entry only once in the process, it calculates all calculations ranging environment correction to Uncertainty calculations on just entering the raw data. PDF is published on click of generate button in draft and in final after approval by lab incharge with a single click on Generate button. In addition to the above, it has also provided feature to pull the data from similar job within the same SRF or at later date, it pulls the data from last calibration the UUC has gone through in this lab. SmartCalibration is perfect solution for commercial calibration lab that could not only reduce the cost but also improve the quality of deliverables.
Any other solution: Not come across solution that is different from the listed above, since this is a blog, I welcome you to comment and also let us know how you are planning to manage cost and quality in the new governance framework.
Related topics to this blog:
NABL ULR guideline and impact on Laboratories and LIMS
https://smallcapcrm.com/SmartCalibration